Researchers have resolved a 50-year-old scientific thriller by figuring out the molecular mechanism that permits tissues to regenerate after extreme harm. The invention may assist information future therapies geared toward lowering the danger of most cancers returning.
Many tissues within the physique, together with the pores and skin and different epithelial layers that line organs, have a outstanding capacity to get well after extreme harm. As a substitute of merely breaking down, they’ll set off a surge of latest cell progress that restores misplaced tissue.
This course of, generally known as compensatory proliferation, was first recognized within the Nineteen Seventies, when researchers noticed that fly larvae may regrow absolutely purposeful wings after their epithelial tissue had been closely broken by high-dose radiation. Since then, related regenerative responses have been noticed throughout a variety of species, together with people, though the underlying molecular mechanisms weren’t properly understood.
When cell dying fuels restoration
New analysis from the Weizmann Institute of Science, revealed in Nature Communications, sheds gentle on how this regeneration happens. The research exhibits that caspases, enzymes greatest recognized for driving cell dying, can even assist sure cells survive and assist tissue restore. By doing so, these cells allow broken tissue not solely to regrow however, in some instances, to change into extra proof against future stress.
The researchers additionally discovered a possible draw back to this course of. The identical survival mechanism could also be exploited by most cancers cells, serving to tumors return in a extra aggressive and treatment-resistant kind. Understanding this pathway may due to this fact inform new methods to enhance wound therapeutic and scale back the danger of most cancers relapse.
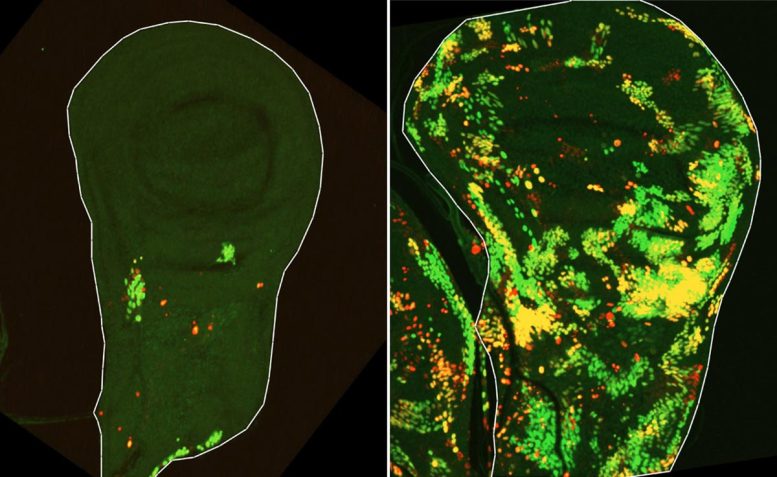
Over the previous twenty years, analysis from many laboratories, together with the group led by Prof. Eli Arama within the Molecular Genetics Division at Weizmann, has proven that caspases are concerned in rather more than cell dying alone. In addition they contribute to important processes that preserve cells and tissues functioning. These findings led Arama, a pioneer in finding out nonlethal roles of caspases, to suspect that these enzymes may also drive compensatory proliferation.
Cells that refuse to die
To check this concept, a staff led by Dr. Tslil Braun in Arama’s lab repeated the basic experiment that first revealed compensatory proliferation by exposing fruit fly larvae to ionizing radiation. This time, nevertheless, they used superior genetic instruments that made it doable to observe tissue regeneration in far higher element.
“We got down to determine cells that push the self-destruct button however survive anyway,” Braun explains. “To do that, we used a delayed sensor that reported on cells during which the initiator caspase had been activated however that however survived the irradiation. That is how we found a inhabitants of cells we named DARE cells. Not solely did these cells survive the irradiation – they multiplied, repaired the broken tissue, and replenished practically half of it inside 48 hours.”
However the researchers additionally wished to know how the remainder of the regenerating tissue contributed to this restoration.
Subsequent, the researchers sought to decipher how DARE cells survive radiation doses that set off apoptosis in neighboring cells.
“We noticed that though the initiator caspase is activated in these cells, the mobile dying course of stops there and doesn’t progress to the subsequent stage,” Arama explains. “We suspected {that a} protein generally known as a molecular motor was liable for this – it may possibly tether the initiator caspase to the cell membrane, stopping it from activating the executioner caspases. Certainly, once we silenced this motor protein, DARE cells proceeded to die and tissue regeneration was impaired. Overactivation of the identical motor protein has beforehand been linked to cancerous tumor progress, which means that this could be one of many mechanisms that allows most cancers cells to evade apoptosis.”
How resistance turns into inherited
It’s recognized that tumors that regrow after radiation remedy typically change into extra aggressive and extra proof against therapy.
“We wished to know whether or not resistance to dying is inherited by the descendants of death-resistant cells that survived the preliminary irradiation,” Arama says. “We discovered that when the identical tissue is irradiated a second time, the variety of cells that die in the course of the first few hours is half that seen after the primary irradiation, and a lot of the lifeless cells belong to the NARE inhabitants. In different phrases, the descendants of DARE cells have been discovered to be exceptionally resistant – seven occasions extra proof against cell dying than cells within the authentic tissue. This will assist clarify why recurrent tumors change into extra resistant after radiation.”

A fragile stability between tissue restore and extra progress is crucial to any regenerative course of. Within the ultimate a part of their research, the researchers revealed how uncontrolled progress is prevented throughout tissue restore after harm. “DARE cells promote the expansion of close by NARE cells, apparently by secreting progress alerts,” Arama notes. “In flip, NARE cells secrete alerts that inhibit the expansion of DARE cells. In actual fact, we have found a negative-feedback loop between the 2 cell populations that stops overgrowth.”
Reference: “Apoptosis-resistant cells drive compensatory proliferation through cell-autonomous and non-autonomous features of the initiator caspase Dronc” by Tslil Braun, Naama Afgin, Lena Sapozhnikov, Ehud Sivan, Andreas Bergmann, Luis Alberto Baena-Lopez, Keren Yacobi-Sharon and Eli Arama, 4 December 2025, Nature Communications.
DOI: 10.1038/s41467-025-65996-2
This analysis was supported by a grant from the Israel Science Basis (grant No. 1378/24) and a grant from the European Analysis Council beneath the EU’s Seventh Framework Program (FP/2007-2013)/ERC grant settlement (616088).


