U.S.-based 3D printer producer 3D Programs, in partnership with French MedTech agency TISSIUM, has secured FDA approval for a bioabsorbable, 3D printed system designed to deal with peripheral nerve harm. This approval validates the polymer’s scientific effectiveness and paves the way in which for its use throughout a variety of medical therapies.
Additive Manufacturing Benefit: Aerospace, Area & Protection is subsequent week, be taught from trade leaders at this sooner or later on-line occasion. Last free registration spots – safe yours now.
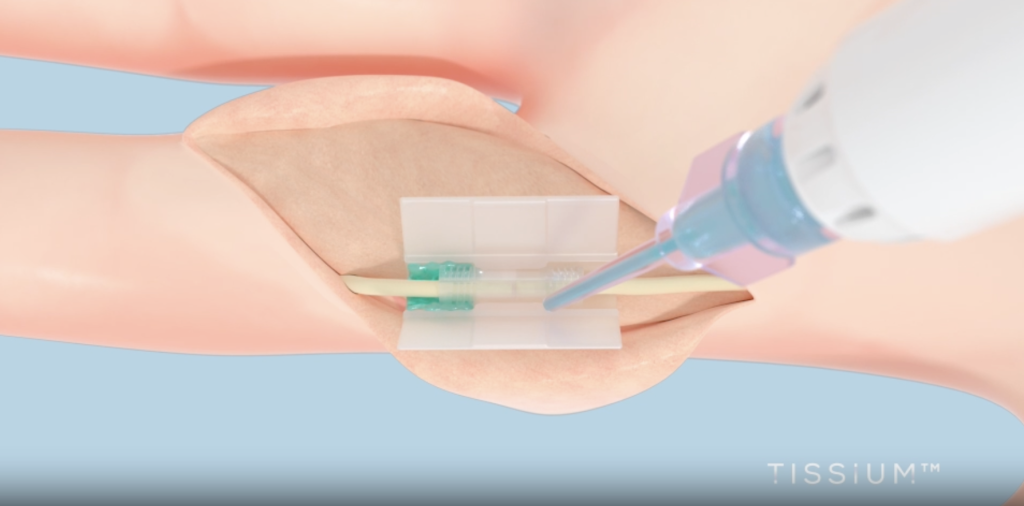
Breakthrough in Peripheral Nerve Restore
For a number of years, 3D Programs and TISSIUM have collaborated to develop a tailor-made 3D printing strategy for repairing broken peripheral nerves. Combining TISSIUM’s proprietary biomorphic polymers with 3D Programs’ bioprinting know-how, they created COAPTIUM CONNECT with TISSIUM Gentle — a totally bioabsorbable, photopolymer-based medical system. This system is the primary of its form: a sutureless, atraumatic resolution that facilitates peripheral nerve restore.
3D Programs acknowledged that due to the polymer’s distinctive properties, the system permits the creation of high-resolution, elastomeric, biodegradable implants which might be unmatched within the trade.
“This can be a vital development in affected person care,” stated Scott Turner, vp of superior methods at 3D Programs. “It has been tremendously rewarding to work alongside the proficient staff at TISSIUM to design an entire 3D bioprinted resolution that gives the potential for sufferers to recuperate from peripheral nerve harm.”


3D Programs’ Broader Regenerative Drugs Imaginative and prescient
This FDA approval enhances 3D Programs’ intensive work in regenerative medication. Since 2017, the corporate has collaborated with United Therapeutics to create a vast provide of human lungs that don’t require immunosuppression, enabling all sufferers with end-stage lung illness to obtain transplants and dwell longer, more healthy lives. Central to this work is 3D Programs’ Print to Perfusion course of, which produces high-resolution scaffolds able to being seeded with residing cells to type purposeful tissue. By combining superior bioprinting methods with biocompatible supplies and a number of cell sorts—together with patient-derived cells—the corporate is delivering customized, residing tissues.
“3D Programs is making a profound influence not solely on how healthcare is delivered, however on the standard of sufferers’ lives, and continues to solidify what I consider is an unparalleled function we play in advancing medication with additive manufacturing functions. This newest accomplishment by TISSIUM, enabled by our distinctive 3D printing know-how, is yet one more instance of how 3D Programs is remodeling affected person look after a greater future,” stated Dr. Jeffrey Graves, president & CEO, 3D Programs.


Wanting forward, 3D Programs goals to capitalize on the worldwide 3D bioprinting market, which is projected to surpass $2.4 billion by 2029. The corporate has supported greater than 150,000 patient-specific surgical procedures and manufactured over two million implants and devices from FDA-registered, ISO 13485-certified services in each the U.S. and Europe.
Latest FDA Approvals in 3D Printing Medical Improvements
Along with the FDA approval of the 3D printed nerve restore system by 3D Programs and TISSIUM, a number of different initiatives have just lately obtained FDA clearance.
In March 2025, Triastek, a Chinese language pharmaceutical firm specializing in 3D printing, introduced that its proprietary 3D printed non-vitamin Okay antagonist oral anticoagulant (NOAC), T20G, obtained Investigational New Drug (IND) clearance from the U.S. Meals and Drug Administration as of February 27, 2025. This regulatory milestone follows an earlier IND approval granted by China’s Nationwide Medical Merchandise Administration (NMPA) in January 2024.
In 2024, 3D Programs additionally obtained 510(okay) clearance from the Meals and Drug Administration (FDA) for its multi-material, 3D printed denture providing. Launched final February, the jetted, monolithic (one-piece) dentures are being billed as a “first-to-market” product that gives unparalleled break resistance and optimum aesthetics. The novelty of this providing lies within the integration of 3D Programs’ NextDent Jet Denture Tooth and NextDent Jet Denture Base supplies right into a single denture resolution. It additionally leverages the corporate’s MultiJet Printing (MJP) know-how, software program and companies.
Be part of our Additive Manufacturing Benefit (AMAA) occasion on July tenth, the place AM leaders from Aerospace, Area, and Protection come collectively to share mission-critical insights. On-line and free to attend. Safe your spot now.
Who gained the 2024 3D Printing Business Awards?
Subscribe to the 3D Printing Business e-newsletter to maintain up with the newest 3D printing information.
It’s also possible to comply with us on LinkedIn, and subscribe to the 3D Printing Business Youtube channel to entry extra unique content material.
Featured picture reveals COAPTIUM CONNECT with TISSIUM Gentle. Picture through TISSIUM.


